免疫疗法创新药取得可喜成果
美国创新药企业Imunon
加强与普利合作
Remarkable results achieved on Innovative Immunotherapy Drugs
Strengthen the cooperation between Poly and US innovative pharmaceutical company Imunon
海南普利制药股份有限公司与美国一家领先的创新药企Imunon,Inc.,展开了一项深入的合作,助力CDMO的一种新型免疫疗法药物,用于巢癌的治疗。
Hainan Poly Pharm. Co., Ltd. has launched a deep cooperation with Imunon, Inc., a leading innovative pharmaceutical company in the United States to assist CDMO in developing a new immunotherapy drug for the treatment of ovarian cancer.
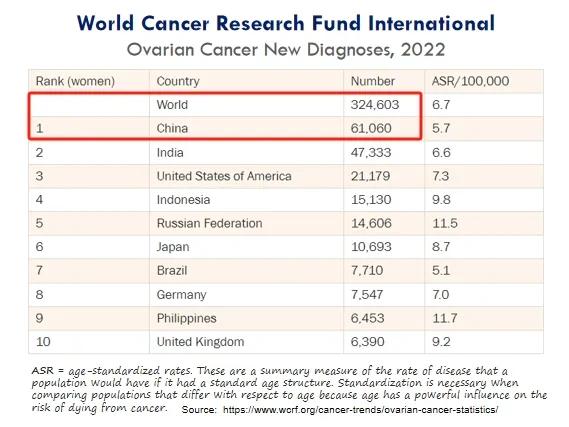
巢癌是指生长在巢上的恶性肿瘤,全球每年新增确诊病例超过30万,中国每年新增确诊病例超过6万,居世界首位。
Ovarian cancer refers to a malignant tumor that grows on the ovaries, with over 300,000 new confirmed cases worldwide and over 60,000 new confirmed cases in China each year, ranking first in the world.
(巢癌年新增确诊病例情况)
(Annual new confirmed cases of ovarian cancer)
由于巢癌早期缺少症状,即使有症状也不特异,筛查的作用又有限,因此早期诊断比较困难,就诊时80%已为晚期(III/IV),而晚期病例又疗效不佳,5年内的死亡率超过60%,死亡率超过宫颈癌及内膜癌之和,高居妇科癌症首位。
Due to the lack of symptoms and no specific symptoms in the early stages of ovarian cancer, plus the limit of screening, the early diagnosis is difficult, and 80% of patients are already in the advanced stage (III/IV) at the time of diagnosis. However, the treatment efficacy in the advanced cases is poor, with mortality rate over 60% within 5 years, which is higher than the sum of cervical cancer and endometrial cancer, ranking first among gynecological cancers.
几十年来,巢癌的标准治疗方案一直停滞不前,手术和化疗是巢癌治疗的主要手段。手术治疗方面,中晚期患者应进行肿瘤细胞减灭术以最大程度切除肉眼可见的肿瘤。化疗方面,一线化疗以紫杉醇联合卡铂为首选。此外,用于维持治疗的靶向药物(抗血管生成药物:贝伐珠单抗、PARP抑制剂:奥拉帕利、尼拉帕利)也为巢癌治疗提供了更多的选择。
For decades, the standard treatment plan for ovarian cancer has been stagnant, with surgery and chemotherapy being the main treatment. In terms of surgical treatment, patients in the advanced stages should undergo tumor cell reduction surgery to remove visible tumors to the greatest extent possible. In terms of chemotherapy, paclitaxel combined with carboplatin is the preferred first-line chemotherapy. In addition, targeted therapy for maintenance therapy (anti angiogenic drugs: bevacizumab, PARP inhibitors: olaparib, niraparib) also provides more options for the treatment of ovarian cancer.
尽管做出了这些努力,但总体生存率(OS)的改善仍然难以实现。
Despite these efforts, the improvement in overall survival rate (OS) remains difficult to achieve.
美国创新药企业的新型免疫疗法药物有可能突破当今标准一线治疗方案,成为第一个也是唯一一个巢癌免疫疗法。
The new immunotherapy drugs developed by innovative pharmaceutical companies in the United States have the potential to break through today's standard front line treatment protocols and become the first and only immunotherapy for ovarian cancer.
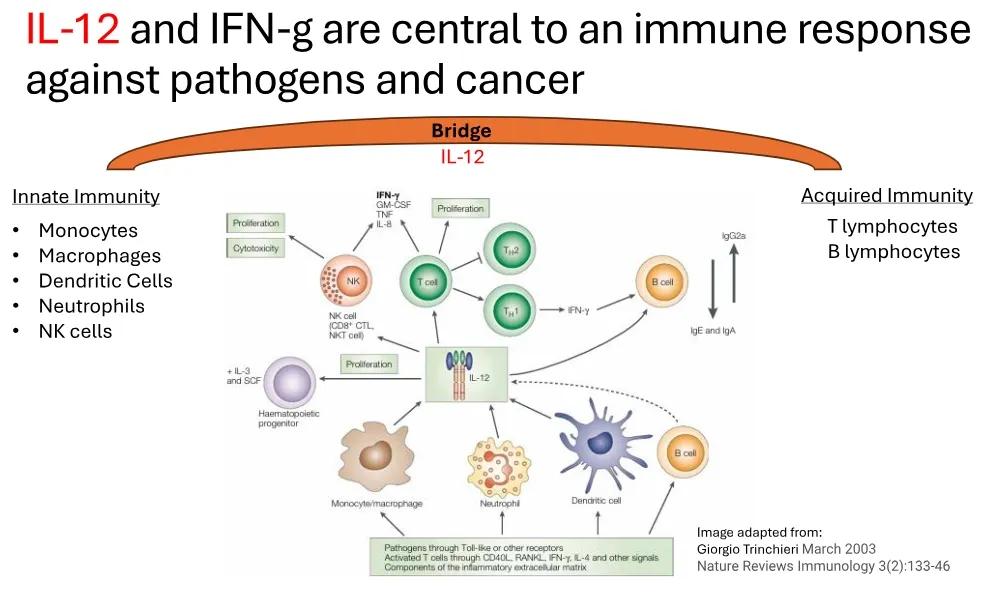
白细胞介素12(IL-12)是免疫反应对抗癌症的核心,可以激活免疫系统对肿瘤进行攻击。Imunon开发的新型免疫疗法药物IMNN-001其纳米颗粒特征可使细胞转染,可局部、可持续地输送IL-12以支持免疫系统对抗巢癌,避免了早期工业界尝试利用IL-12时观察到的系统性毒性。
Interleukin-12 (IL-12) is the core of the immune response against cancer, which can activate the immune system to attack tumors. The nano particle characteristics of the new immunotherapy drug IMNN-001 developed by Imunon enables cell transfection and can locally and sustainably deliver IL-12 to support the immune system in combating ovarian cancer, avoiding systemic toxicity observed with earlier attempts in the industry to harness IL-12.
在之前的合作中,普利制药向美国合作伙伴提供了高质量的临床批样品,这些样品已被用于早期临床试验中,且取得了优异结果。
In previous cooperation, Poly Pharm. has provided high-quality clinical batch samples to its American partners, which have been used in early clinical trials and achieved excellent results in early stage clinical trials.
基于双方对彼此专业能力的高度认可和信任,双方已进一步扩大合作范围,以实现更大的协同效应。普利制药将承担该新药项目新制剂工艺改进工作,并继续承担后续临床样品生产任务。
Based on the high recognition and trust of each other's professional abilities, both parties have further expanded the scope of cooperation to achieve greater synergies. Poly Pharm. will continue to improve the process for the new drug and the subsequent clinical sample production.
2期临床试验结果达业内领先水平
Phase II clinical trial results reach industry-leading leve
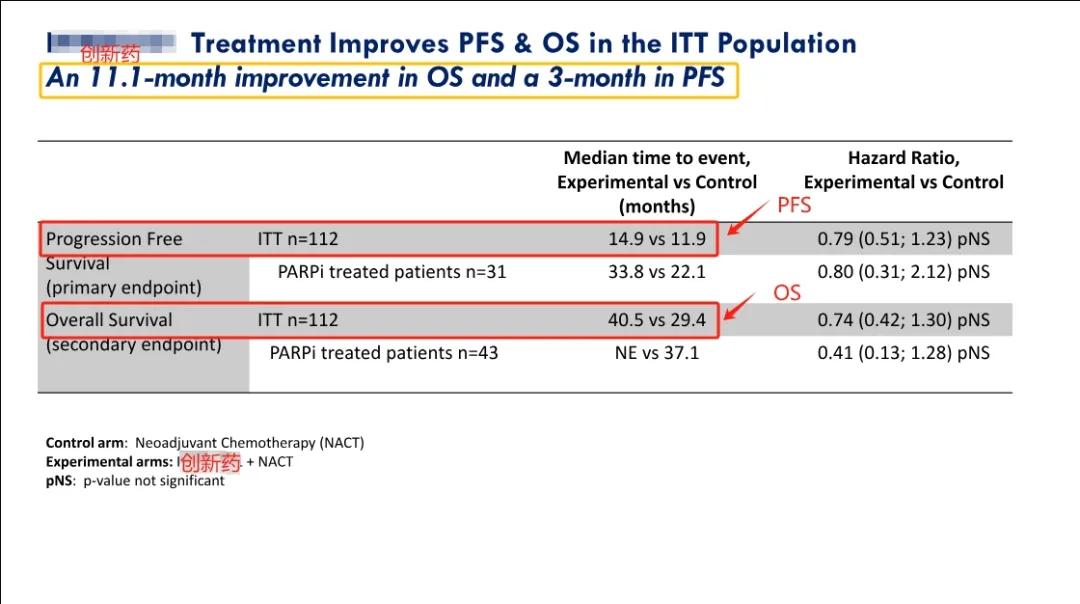
近期,IMNN-001的2期临床试验结果已被公布,这种新型免疫疗法药物在治疗组中的表现显著优于标准治疗方案,如果在3期临床试验成功,预计将取代标准治疗,成为新的首选一线治疗方案。研究结果如下:
Recently, the Phase II clinical trial results of IMNN-001 this innovative drug have been announced which shows better performance than the standard treatment in the treatment group and if successful in Phase 3 is expected to replace the standard treatment as a new first-line treatment regimen. The research results are as follows:
1)与单独采用标准治疗相比,意向治疗人群 (ITT) 的中位总生存期 (OS) 增加11.1个月。其中,对于接受了PARP抑制剂治疗的人群,IMNN-001组的患者的中位OS尚未达成(试验组仍然有很多患者存活,因此无法计算出一个确切的中位OS值,这种情况通常表明试验组的治疗效果较好),其中部分女性患者存活已经达到5年。与标准治疗组相比,这些女性使用IMNN-001治疗的总生存期(OS)提高了144%。
1) Compared with standard treatment alone, the median overall survival (OS) of the intention to treat population (ITT) increased by 11.1 months. Among them, for the population receiving PARP inhibitor treatment, the median OS was not yet reached in women receiving IMNN-001 treatment, with some women approaching the 5-year mark. . Compared to the standard treatment group, the improvement in OS with IMNN-001 in these women was 144%.
2)接受≥3剂新型免疫疗法药物治疗的患者的中位 OS 增加了15.7个月。
2) The median OS of patients receiving ≥3 doses of new immunotherapy drugs increased by 15.7 months.
3)与单独的标准治疗相比,PFS (无进展生存期)有3个月的改善。
3) Compared with standard treatment alone, PFS (progression free survival) showed a 3-month improvement.
鉴于以上积极的临床试验结果,合作双方对3期临床试验和商业化都充满了信心。
Given the clinical trial results mentioned above, both parties are confident in the Phase III clinical trial and commercialization.
(Imunon公司合作伙伴到访普利浙江杭州基地,并表示感谢和对公司的认可)
(Visit Hangzhou Site (Zhejiang) )

(Imunon公司合作伙伴到访普利海南海口基地,同公司人员一同参观实验室、工厂等)
(Visit Haikou Site (Hainan) )

此外,在该新药通过临床试验并获得相关监管部门的批准后,普利制药将负责该药的商业化生产,确保药品能够顺利进入市场,满足广大患者的需求。通过此次合作,双方将共同推动该新药项目的成功开发和市场推广,为更多的患者带来福音。
In addition, once the registration clinical trials are successfully completed and marketing authorization obtained from relevant regulatory authorities, Poly Pharm. will be responsible for the commercial production of the drug to ensure its entry into the market and meet the needs of many patients. Through this collaboration, both parties will jointly promote the successful development and market promotion of the new drug project, bringing good news to more patients.
未来,该药品有望在中国进行申报,双方将进一步合作共同开拓中国市场。同时,普利制药也将努力推动该药物在海南博鳌乐城国际医疗旅游先行区的落地(位于海南自贸港东南边陲小镇博鳌的乐城先行区拥有独一无二的特许政策:国家允许进口使用已在境外上市、但未在中国批准注册的药品和医疗器械,让国人不出国门就能享受国际前沿医疗服务),将创新药引进中国,造福国内患者。
The drug is expected to be submitted in China in the future, and both parties will further cooperate to jointly explore the Chinese market. At the same time, Poly Pharm. will also strive to promote the landing of the drug in Hainan Boao Lecheng International Medical Tourism Pioneer Zone (Lecheng Pioneer Zone, located in Boao, a small town in the southeast of Hainan Free Trade Port, has a unique licensing policy: it allows the import and use of drugs and medical devices that have been listed overseas but not approved for registration in China, so that the citizen can experience international cutting-edge medical services without leaving the city), and introduce innovative drugs to China to benefit domestic patients.
关于普利制药
About US

普利制药 1992 年成立于海口,是中国医药制剂国际化先导企业和国家工信部智能制造示范企业,已被国家工信部纳入工业转型升级中国制造 2025 年儿童药重点项目企业,2023年海南普利通过海关“AEO”高级认证。
Poly Pharmaceuticals was established in Haikou in 1992 and is a pioneer enterprise in the internationalization of Chinese pharma-ceutical preparations and a demonstration enterprise for intelligent manufacturing by the Ministry of Industry and Information Technology of China. It has been included by the Ministry of Industry and Information Technology in the key projects for the industrial transformation and upgrading of "Made in China 2025" for chien's medicines. In 2023, Hainan Poly passed the "AEO" advanced certification of customs.
此前,海南普利及其子公司浙江普利、安徽普利也曾多次顺利通过美国FDA、欧盟EMA现场审计。作为中国医药制剂国际化先导企业,普利制药多年来一直恪守全球较高质量标准,是国内为数不多的原料药和注射剂研发和生产平台,也是为数不多的同时获得美国、中国、欧盟等药监部门批准的原料药、关键辅料、药物药剂和GMP中间体CMO/CDMO的优质供应商。
Previously, Hainan Poly and its subsidiaries, Zhejiang Poly and Anhui Poly, have also successfully passed on-site audits by the U.S. FDA and the European Union EMA. As a pioneer enterprise in the internationa-lization of Chinese pharmaceutical pre-parations, Poly Pharmaceuticals has adhered to high global quality standards for many years. It is one of the few platforms in China for the research and development and production of APIs and injectables, and it is also one of the few high-quality suppliers that have been approved by regulatory agencies such as the FDA in the United States, the NMPA in China, and the EMA in the European Union for APIs, key excipients, pharmaceutical preparations, and GMP intermediates CMO/CDMO.
本文作者可以追加内容哦 !

